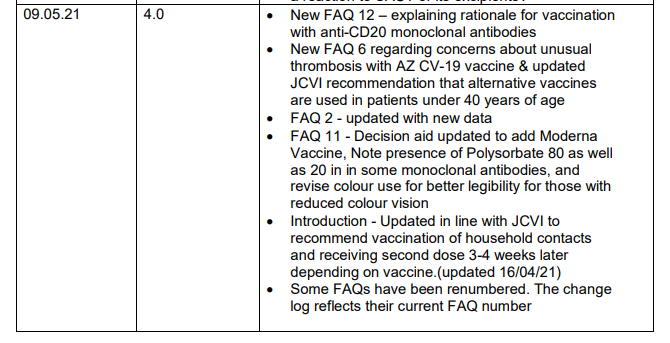
UPDATE – Clinician FAQs and guidance on COVID-19 vaccine for patients receiving SACT – Version 4.0
Note that version 4.0 of ‘Clinician FAQs and guidance on COVID-19 vaccine for patients receiving SACT’ is now available on the UKCB website here: https://www.ukchemotherapyboard.org/publications.
Updated for this version: